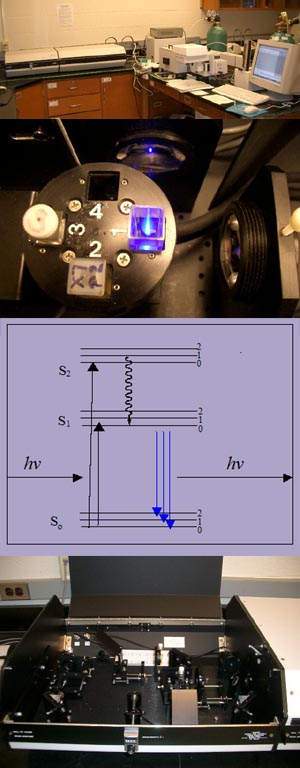
 A PTI Steady State Fluorometer System, consisting of an arc lamp and a photomultiplier detection system (top) is used to take fluorescence excitation and emission spectra measurements of fluorophore doped aerogels and xerogels in the Aerogels Lab. Fluorescence occurs when molecular species absorb energy from light waves that match the quantized energy levels of that species. The theory behind fluorescence is shown in the Jablonksi diagram (middle). The energy level labeled S0 represents the ground state of the fluorophore; the levels labeled S1 and S2 represent the excited states. The straight arrows that point up designate the promotion of a molecule to an excited state by absorption of light. The curved arrow that points down is a representation of the internal conversations that excited molecules undergo in order to reach the lowest excited state level. The straight arrows that point down indicate fluorescence; it is the process by which an excited species at S1 emits a photon to return to its ground state at S0. These energy levels are characteristic to each chemical species; therefore, the wavelengths of the absorbed and emitted light are also characteristic to each chemical species. Fluorescence lifetime is essentially the average time it takes for the excited species to release its energy through fluorescence and return to its ground state.
A PTI Steady State Fluorometer System, consisting of an arc lamp and a photomultiplier detection system (top) is used to take fluorescence excitation and emission spectra measurements of fluorophore doped aerogels and xerogels in the Aerogels Lab. Fluorescence occurs when molecular species absorb energy from light waves that match the quantized energy levels of that species. The theory behind fluorescence is shown in the Jablonksi diagram (middle). The energy level labeled S0 represents the ground state of the fluorophore; the levels labeled S1 and S2 represent the excited states. The straight arrows that point up designate the promotion of a molecule to an excited state by absorption of light. The curved arrow that points down is a representation of the internal conversations that excited molecules undergo in order to reach the lowest excited state level. The straight arrows that point down indicate fluorescence; it is the process by which an excited species at S1 emits a photon to return to its ground state at S0. These energy levels are characteristic to each chemical species; therefore, the wavelengths of the absorbed and emitted light are also characteristic to each chemical species. Fluorescence lifetime is essentially the average time it takes for the excited species to release its energy through fluorescence and return to its ground state.
A PTI Laser Strobe Fluorescence Lifetime System that uses pulsed Nitrogen and dye lasers (bottom) is used to take fluorescence lifetimes of the aerogel and xerogels. The Nitrogen Laser delivers a pulse at 337 nanometers with a pulse width of 600 picoseconds, that results in a pulse energy of 1.45 millijoules. The Dye Laser delivers a very narrow 0.04 nanometer output from 360 to 900 nm with 220 microjoules of energy per pulse at 500 nm.