Here are some sets of drawings that I did for a variety of projects a long time ago. Note that the artwork is rather crude, and some of the labels, captions, and even the spelling exhibit a somewhat primitive understanding of petrology and English. Nonetheless, I hope you gather that drawings can frequently offer insight of a process or views that are difficult to capture with a photo or computer-rendered illustration.
Figures done in the 1977 Petrology class with Peter Robinson, University of Massachusetts, Amherst, Hawaii suite
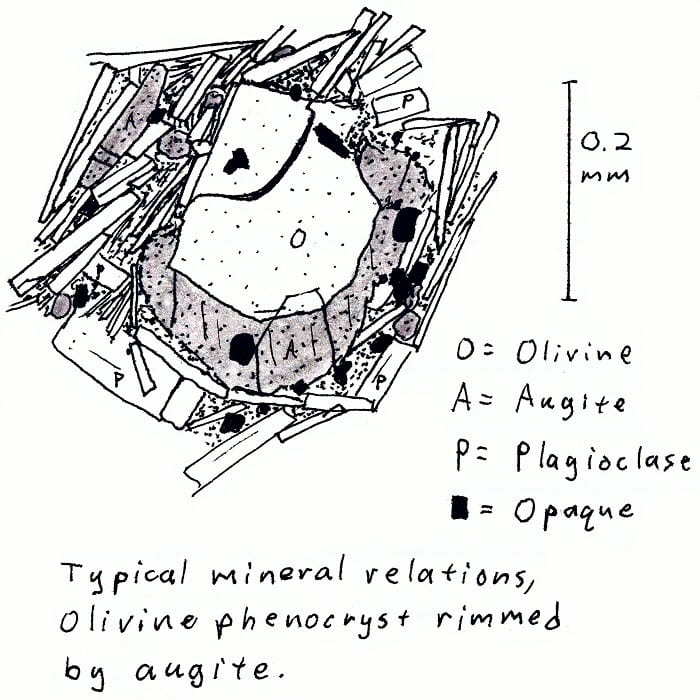
HW-2A. Hawaii alkali basalt.
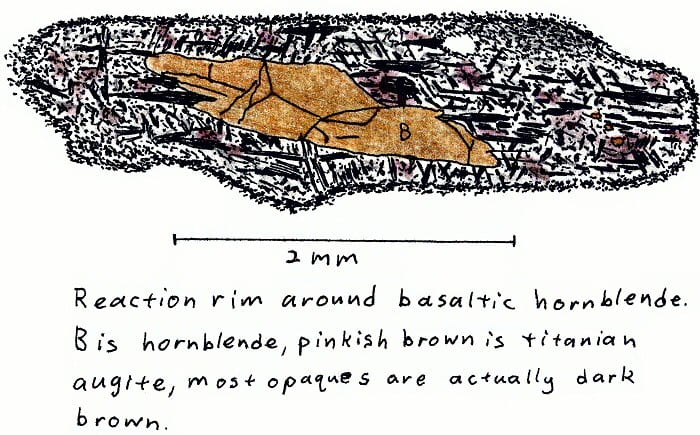
HW-2A. Hawaii alkali basalt.
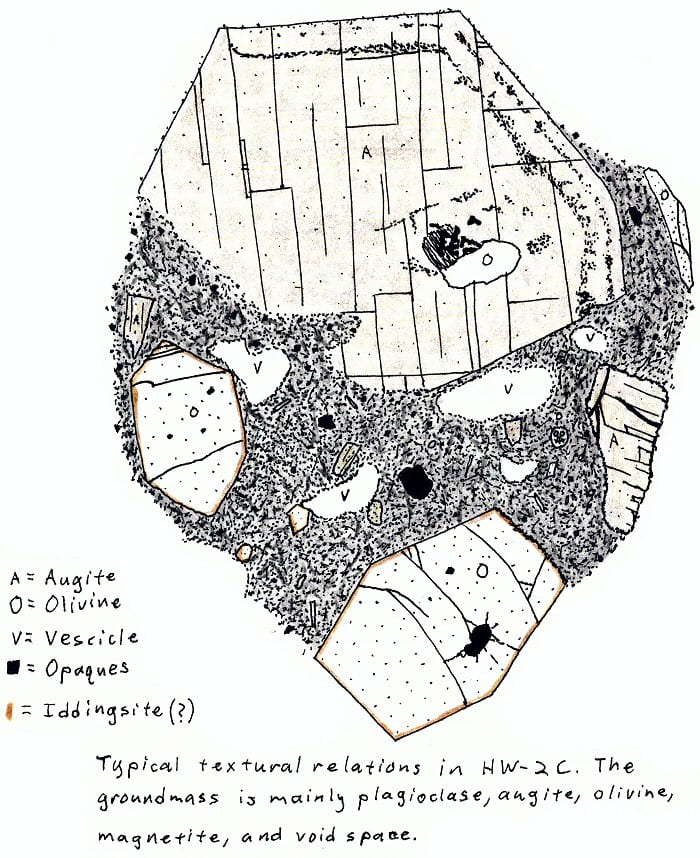
HW-2C. Hawaii alkali olivine basalt.
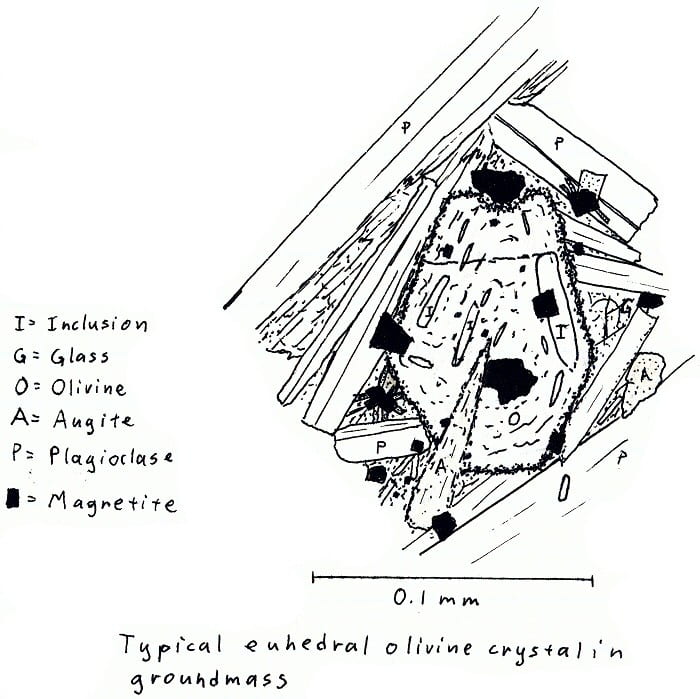
HW-3. Hawaii olivine basalt.

HW-3. Hawaii olivine basalt.
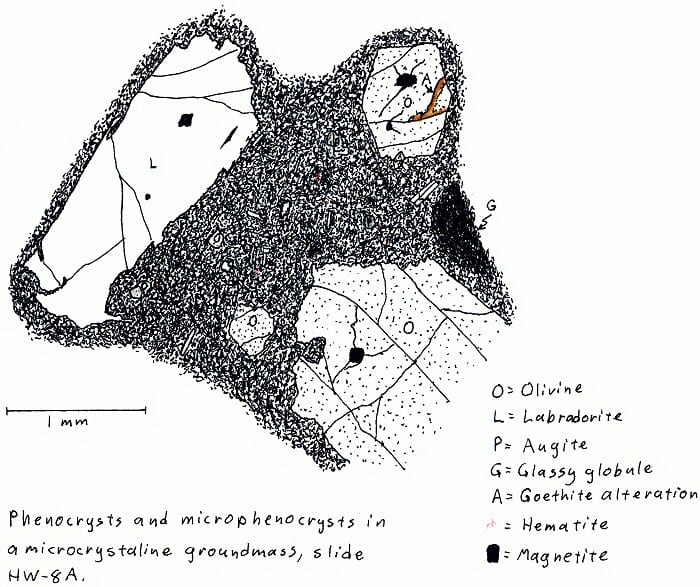
HW-8A. Hawaii olivine basalt.
HW-8B. Hawaii olivine basalt.

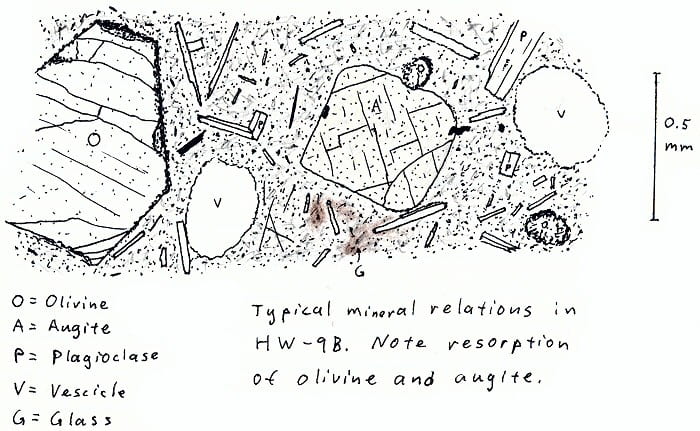
HW-9B. Hawaii olivine basalt.
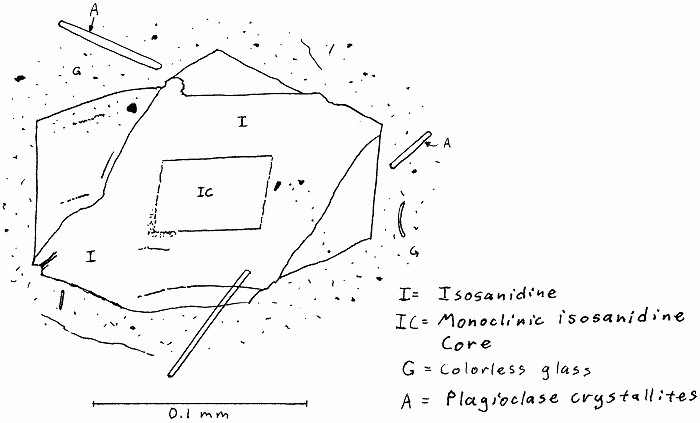
HW-11A. Hawaii trachyte obsidian.
p.s. I don’t think isosanidine is a real thing.
Figures done in the 1977 Petrology class with Peter Robinson, University of Massachusetts, Amherst, Salem suite
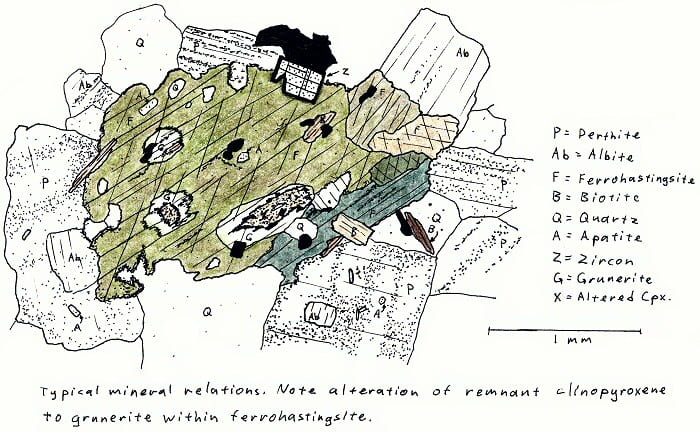
SA-4A. Alkali fayalite granite. No fayalite here, though.

SA-4B. Alkali fayalite granite. Here is fayalite.
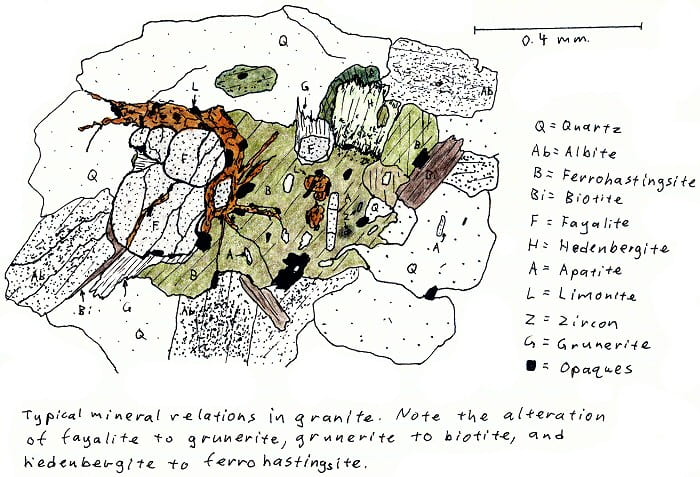
SA-4D. Alkali fayalite granite. More fayalite.
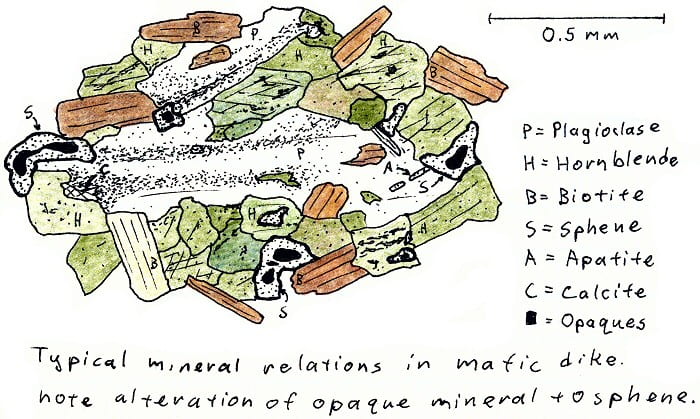
SA-4D. Basalt dike cutting granite, metamorphosed by cooling in contact with hot granite.
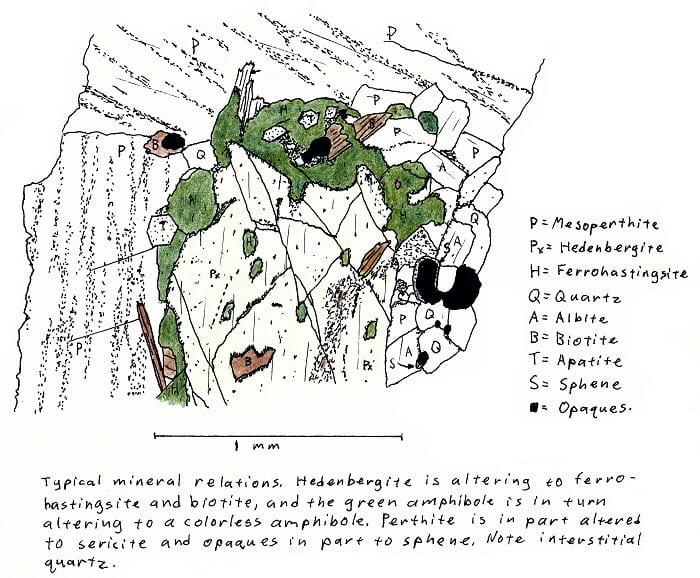
SA-5. Hedenbergite granite.
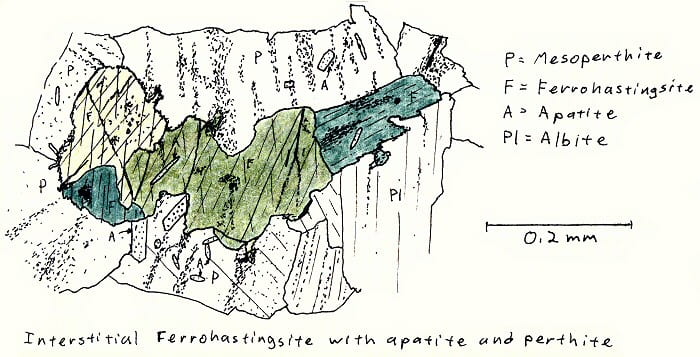
SA-6B. Alkali granite.

SA-6B. Alkali granite.
Figures done in the 1977 Petrology class with Peter Robinson, University of Massachusetts, Amherst, assorted rocks
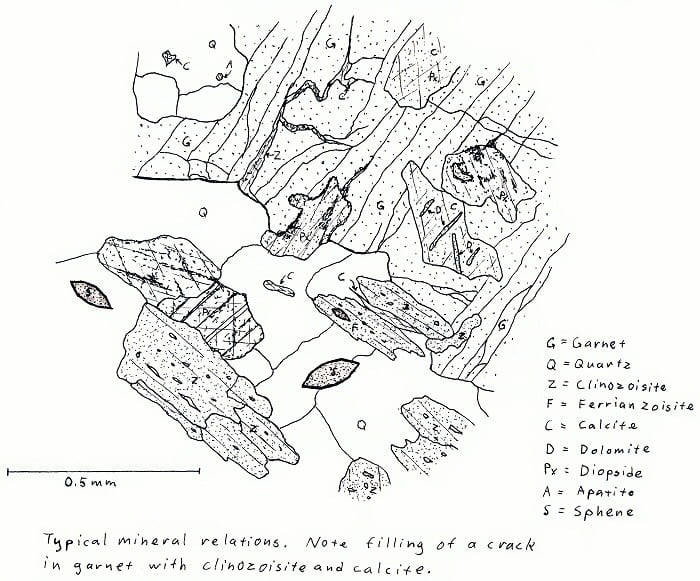
7Q5. Amphibolite facies calc-silicate rock. Not very colorful, except in cross-polarized light.
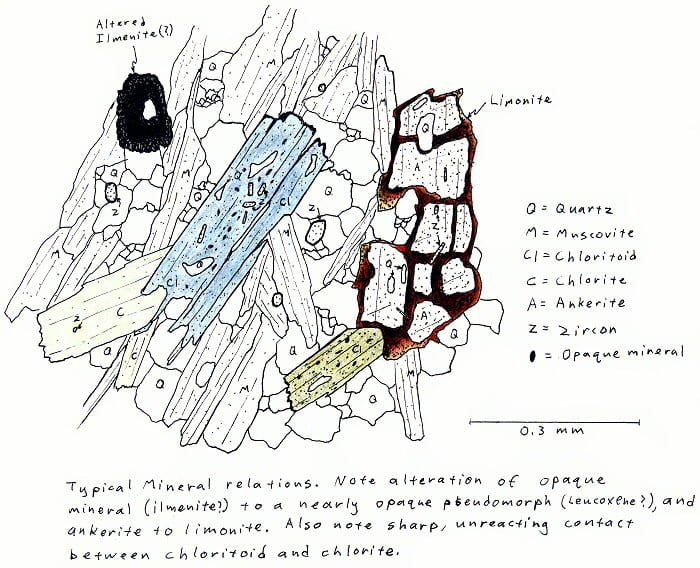
CHL. Greenschist facies chloritoid-chlorite-muscovite schist.
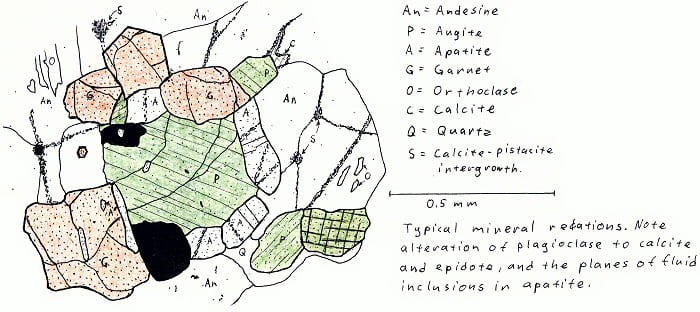
Elizabethtown, NY. Two-pyroxene granulite (only one pyroxene illustrated here).
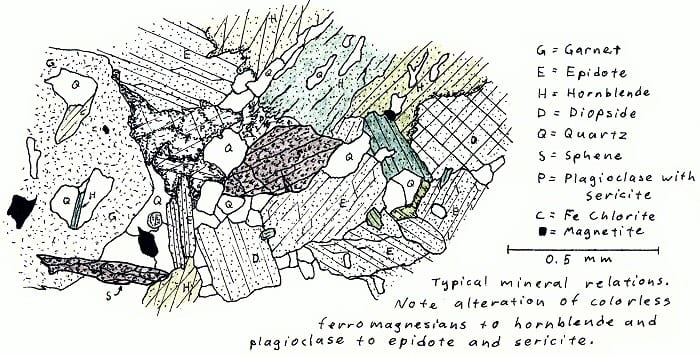
O5F8. Amphibolite facies calc-silicate rock.
p.s. Sphene forever!

UO1B. Amphibolite facies pelitic schist.
Masters thesis illustrations on retrograded pelitic schists, New Salem area, Massachusetts
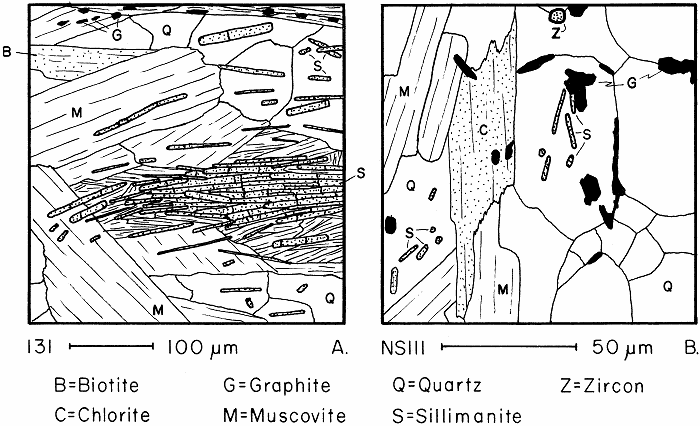
Sillimanite in slightly and severely retrograded schists.
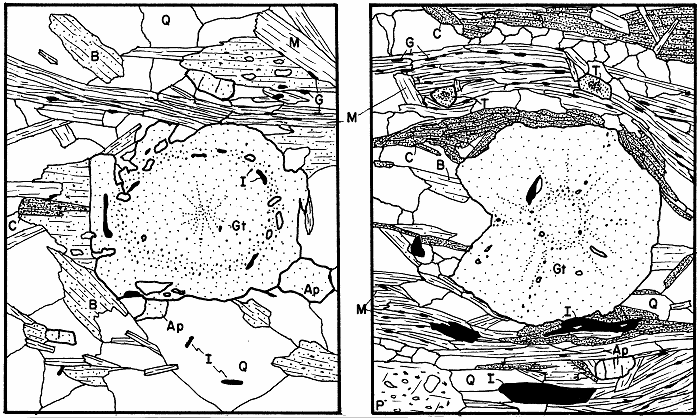
Garnet in two slightly retrograded rocks, first two examples.
Garnet in two severely retrograded rocks, second two examples.

Fresh vs. retrograded and completely pseudomorphed staurolite.

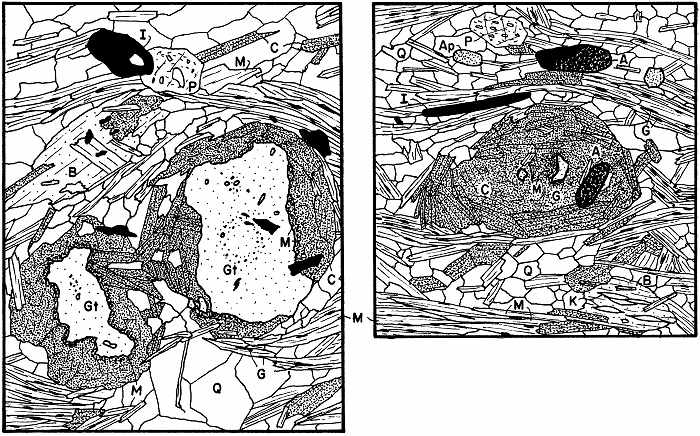
Partly retrograded garnet, with retrograde chloritoid. See illustration below.
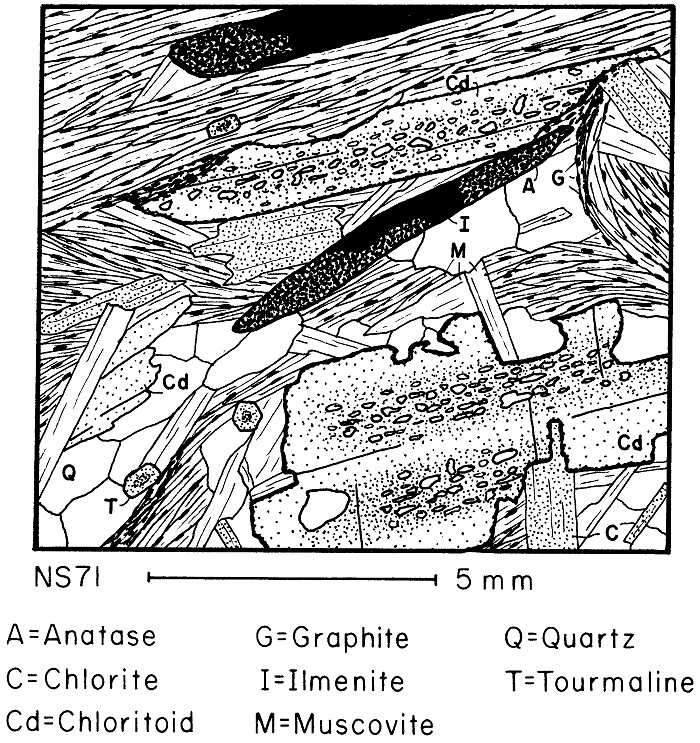
Chloritoid in partially retrograded schist, also showing ilmenite partly retrograded to anatase.
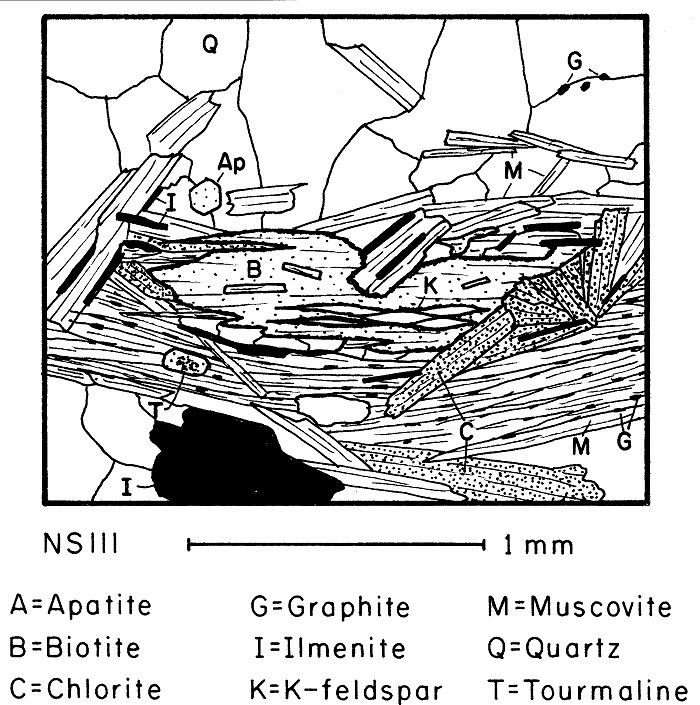
Partially retrograded biotite containing lenses of retrograde K-feldspar. The blurry but apparently small 2V of the K-feldspar suggests it is sanadine.
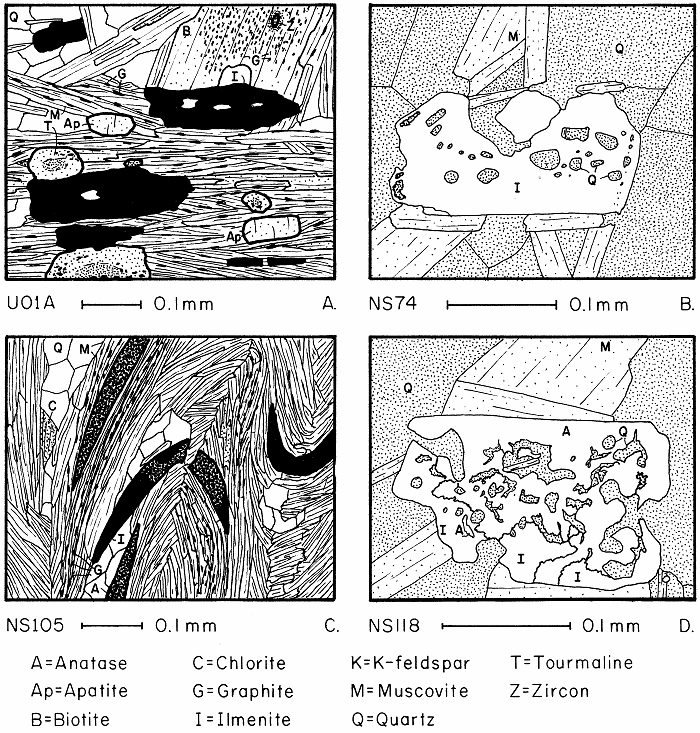
Prograde ilmenite and retrograde anatase in transmitted (left) and reflected (right) light.

Sphene, ilmenite, and anatase, plus analytical data.
In case you forgot: Sphene forever!
Spots on garnet analyzed by electron microprobe, from Hollocher, 1987
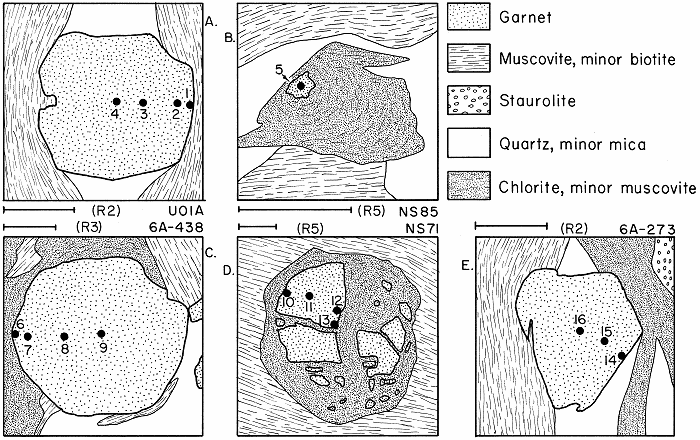
Several garnets showing garnets in various states of retrograding in various relationships to chlorite.
Sketches made for research paper summaries, Volcanology class taught by J.M. Rhodes and Marty Godchaux
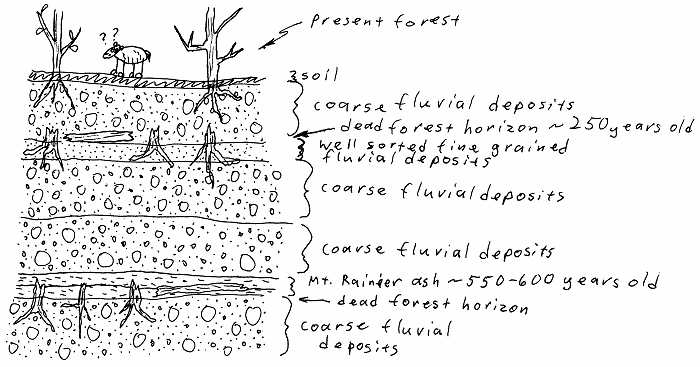
Ash beds in the Mt. Rainier area.
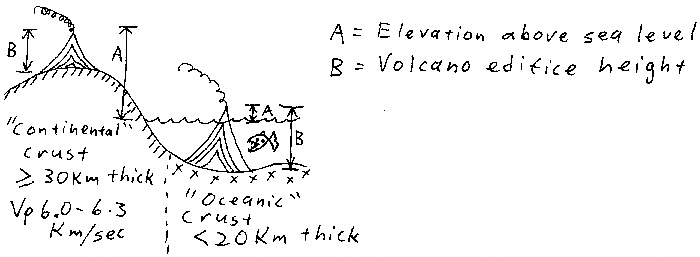
Volcano top elevations, original point unclear to me.

Volcano top elevations based on magma chamber depths.
Sketches made for some 1980’s field trip guidebooks

Orthopyroxene tonalite partial melts in amphibolite, Rt. 9, Belchertown, Massachusetts.

In-situ melt veins in two-pyroxene granulite, Masapaug Rd. near Sturbridge, MA.
Slides for a presentation on retrograde metamorphism, M.S. thesis, circa 1979 (back when I had a sense of humor)
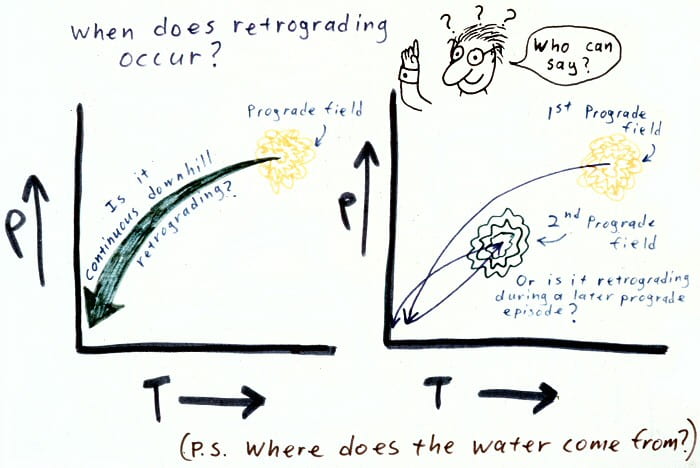
Retrograde metamorphism: during cooling or or more complex polymetamorphism.
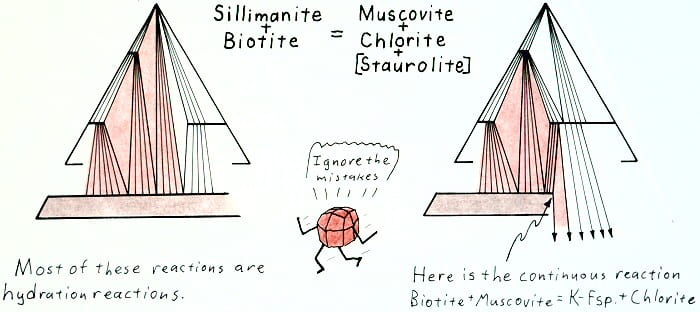
Retrograde sillimanite-out reaction.
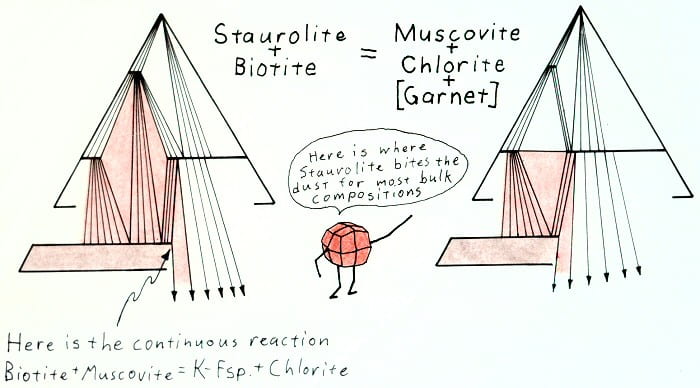
Retrograde staurolite-out reaction.
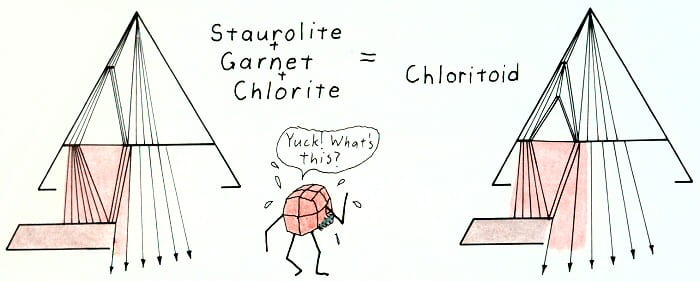
Retrograde chloritoid-in reaction.
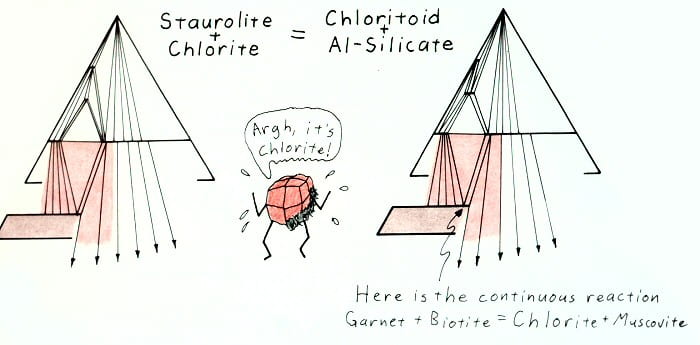
Hypothetical retrograde reaction removing staurolite.

Retrograde hypothetical reaction.

Retrograde hypothetical reaction, terminal for staurolite in this system.

Retrograde garnet-out reaction progression.

Retrograde garnet-out reaction.

Sans gloves, chlorite pseudomorphs after garnet actually look rather like this.
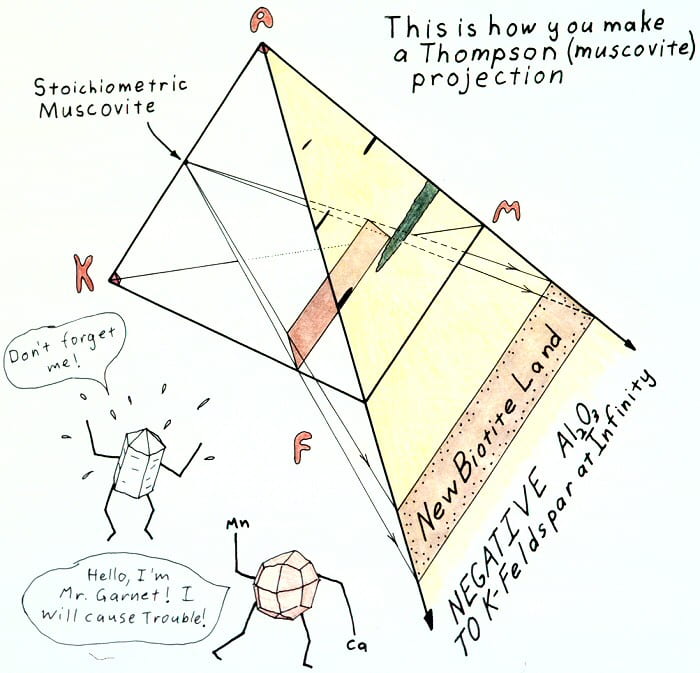
Muscovite projection explanation.
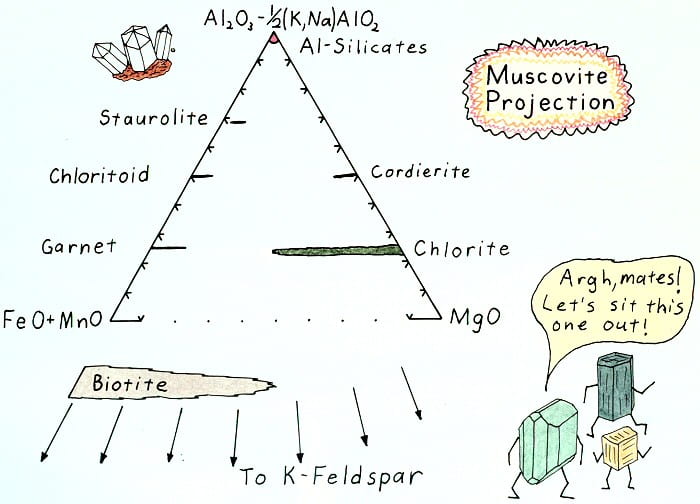
Muscovite projection result.
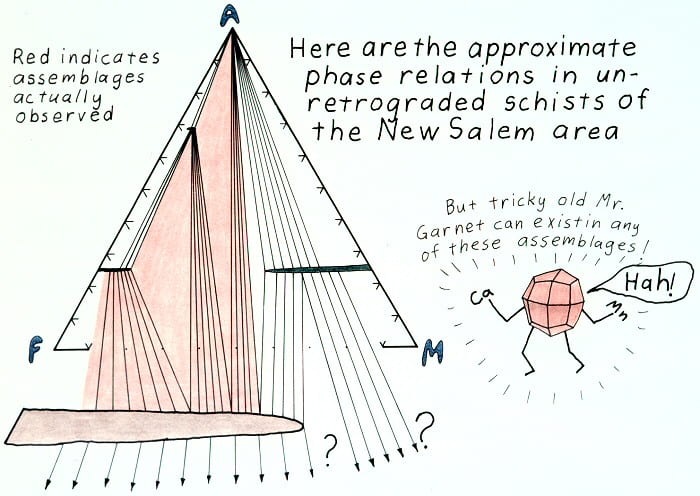
Prograde phase relations.

Edgewise view of the AKFM tetrahedron.
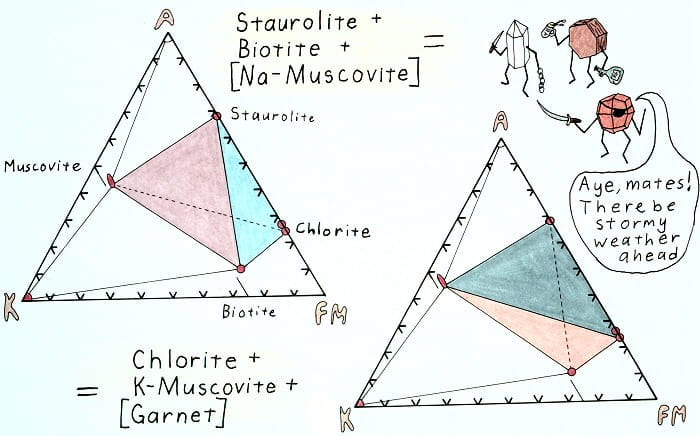
Edgewise view of the AKFM tetrahedron showing the retrograde staurolite-out reaction.
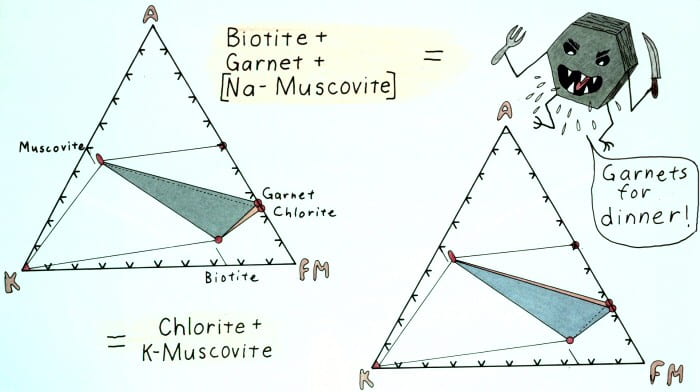
Edgewise view of the AKFM tetrahedron showing the retrograde garnet-out reaction.
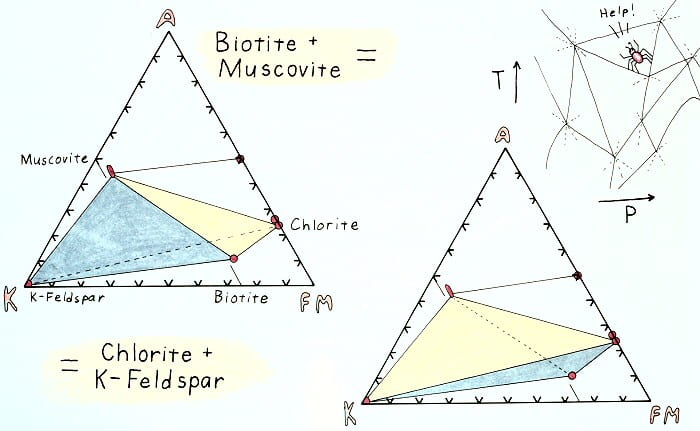
Edgewise view of the AKFM tetrahedron showing the retrograde K-feldspar-in reaction.

One way to think of why garnets occur in so many assemblages.

One way to look at oxide and silicate assemblages. Don’t take this too seriously.

Well, haven’t you been wondering?
