About 30% of people have staph bacteria, usually living commensally on the skin and in the nostrils. Occasionally, staph can break the skin and cause infections ranging in seriousness from small boils to sepsis and pneumonia.
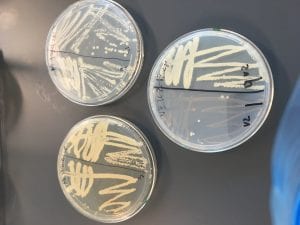
Staphylococcus bacteria are named so because they are round, or coccus shaped, and they are arranged in grape shaped clusters (the Greek word for grapes is transliterated to staphule). On plates, S. aureus usually create yellow or golden colonies. They grow readily between 18 and 40 degrees Celsius, and are faculitative anaerobes, meaning they will use oxygen if available but can live without it.
Staphylococcus aureus is believed to be living peacefully on the skin or in the nose of 1/3 of the world’s population. In 2006, it was found that for every 10,000 visits to the hospital, 410 of those visits were for a staph skin infection such as abscesses and cellulitis; the number of skin infections in general might be much higher due to the relative lack of seriousness of some skin problems like impetigo and boils. Staph bacteremia, or presence of staph bacteria in the blood, is the cause of an estimated 23% of all cases of sepsis, and can lead to infection of the heart and infection of the bones. Staph is also a common complication of pneumonia, and S. aureus is implicated in more than 40% of healthcare acquired pneumonias. As you can see, staph infections can occur from a variety of ways; the best prevention is simply commonsense risk mitigation, such as cleaning open wounds and having good hygiene.
The antibiotic-resistant form of staph that everyone is afraid of is MRSA (methicillin-resistant Staphylococcus aureus). MRSA infections are commonly acquired in health care settings, but up to 12% of MRSA infections now are from the broader community. Oddly, MRSA refers to staph resistant against other antibiotics, though most antibiotics it is commonly resistant to is in the beta-lactam class, which includes methicillin and penicillin. Resistance happens when staph creates the enzyme beta-lactamase to cleave an important bond in the antibiotic. It recently became resistant to vancomycin, the antibiotic doctors usually go for when they treat MRSA. The antibiotics Bactrim, clindamycin, minocycline, and doxycycline are still effective and are still widely prescribed to patients.
Instead of playing with Stapholococcus aureus in the lab, we are using Staphylococcus epidermidis.
https://www.ncbi.nlm.nih.gov/books/NBK441868/
https://www.cdc.gov/mrsa/community/index.html
https://cmr.asm.org/content/10/3/505.long
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4451395/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3367609/
https://en.oxforddictionaries.com/definition/staphylococcus
https://en.oxforddictionaries.com/definition/aureus
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3372331/
https://www.the-hospitalist.org/hospitalist/article/125986/what-best-treatment-adult-patient-staphylococcus-aureus-bacteremia
https://academic.oup.com/femsre/article/41/3/430/3608758
https://www.uptodate.com/contents/methicillin-resistant-staphylococcus-aureus-mrsa-beyond-the-basics